ISO 13485 Software for Quality in the Medical Device Industry
Manufacturers and suppliers of medical technology must adhere to the rules and regulations of ISO 13485, the European Medical Device Regulation (MDR) and the US Food and Drug Administration (FDA). The sophisticated CAQ.Net software solutions enable medical technology companies around the globe to fulfil these stringent rules and regulations and successfully master all associated quality management and validation processes.
Amongst other aspects, the framework conditions that must be adhered to when manufacturing medical technology products include the application of CAPA measures (Corrective And Preventive Actions), the warranty of consistent traceability and change tracking, the implementation of a standard-compliant risk management system, and the deployment of effective training and documentation management processes. In order to be able to successfully comply with these various requirements, companies need software that ensures consistent documentation and comprehensive monitoring, and allows rapid and cross-departmental reactions to any type of change: this software is CAQ.Net.
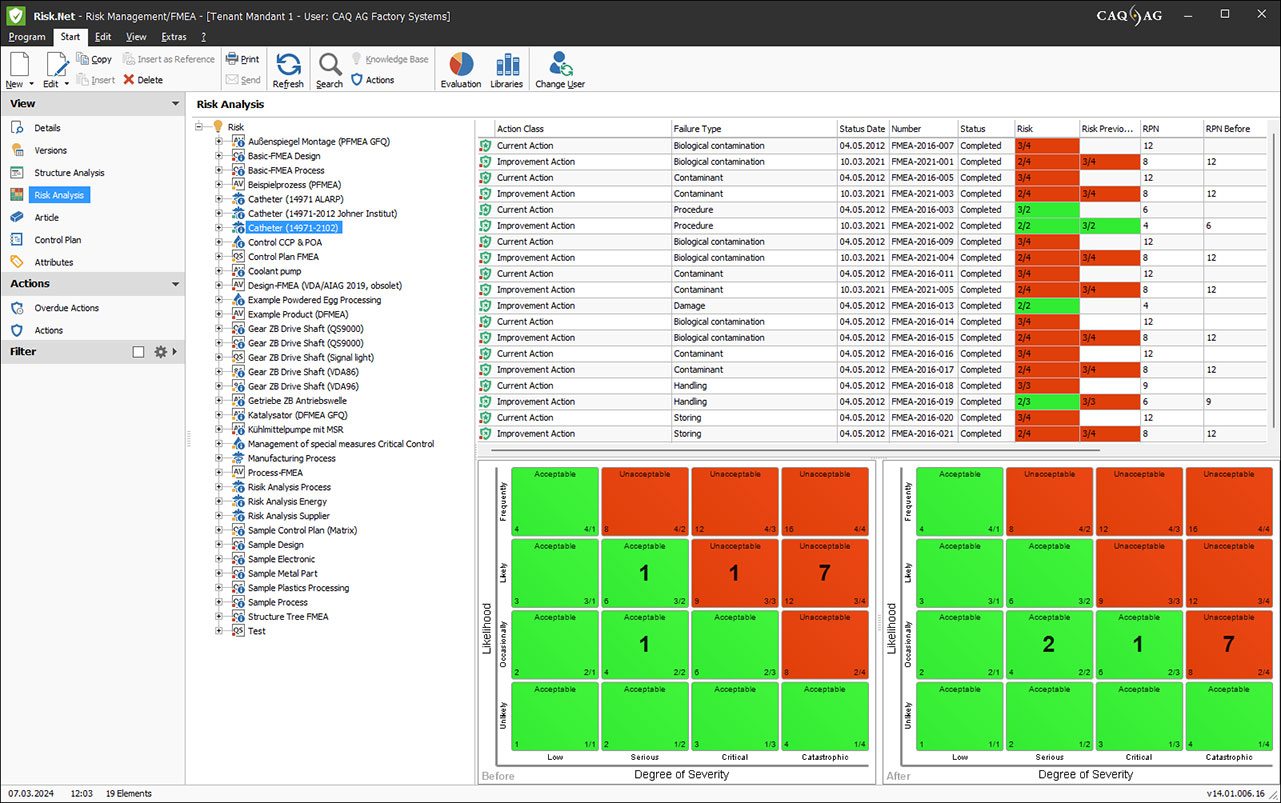 Before/After Matrix according to DIN EN ISO 14971
Before/After Matrix according to DIN EN ISO 14971
Quality Management Software Solutions
CAQ.Net is a user-friendly modular software solution that facilitates the adherence to internal and external requirements as well as regulatory and legal obligations. It allows companies to conduct comprehensive compliance management in a well-structured manner that corresponds to obligations relating to MDR, GxP, FDA, ICH, ISO 13485, 21 CFR 820, ISO 14971, and 21 CFR Part 11.
CAQ AG also works in close cooperation with a variety of partner companies who are specialized in qualification and validation procedures in the medical field. The resulting target/actual comparison between the fully validatable CAQ.Net software and the most current requirements by manufacturers and suppliers of medical technology means that our customers are always in command of a cutting-edge software solution that allows professional, efficient, and standard-compliant quality management.
Most Popular Components of the ISO 13485 Software
CAPA
Initiate CAPA processes with various triggers, such as from gauge management, audit management, or complaint processing, using the assistant-guided functions in CAQ.Net.
Learn more about the
CAPA Assistant »
Risk Management
Identify, evaluate, and document relevant risks and fulfill the requirements of EN ISO 14971.
Learn more about the
Risk Management Software »
Root Cause Analysis
Explore the root causes of problems and uncover potential for improvement using Ishikawa diagrams and the 5-Why method.
Learn more about the
RCA Software »
Document Control
With QBD.Net, every employee always has access to the documents he needs in the current and valid version and can view and document all relevant control steps at any time.
Learn more about the
Document Control Software »
Skills Matrix
Always keep track of your employees qualifications and get automatic reminders when they need to be renewed.
Learn more about the
Skills Matrix Software »
Change Control
Monitor and control all change processes in the company and always keep an eye on the details.
Learn more about the
Change Management Software »
Medical Technology Companies that Rely on CAQ.Net